Menu
Mirpur, Dhaka-1216
Mirpur DOHS, Bangladesh
+88 02 44806853
24/7 Customer Support
sales@cleanwaterbd.com
Mail Us If Emergency
Mirpur DOHS, Bangladesh
24/7 Customer Support
Mail Us If Emergency

The main alternative to chemical wastewater treatment is biological wastewater treatment. Instead of using chemicals to break down waste, this process uses beneficial microorganisms to biodegrade waste material.
Biological wastewater treatment is best at removing contaminants that biodegrade easily. Compounds with simple molecular structures – monomers and dimers, rather than complex polymers – are easiest for microorganisms to digest. Below is a list of contaminants biological wastewater treatments commonly remove:

This technical guide explains what biological wastewater treatment is, how it works, and how it’s used to improve the quality of industrial waste water streams prior to discharge. The guide looks at the main types of treatment including aerobic, anaerobic and anoxic; the importance of Biological Oxygen Demand or BOD, and the different types of waste water treatment technologies currently including various types of bioreactor.
Biological wastewater treatment is usually the second stage in the cleaning process and comes after larger particles have been removed through the filtering or settlement stages.
Biological wastewater treatment is an effective way of breaking down and eliminating organic waste, typical of the waste products produced in the food and drink, chemical, oil and gas industries.
Biological treatment is complex and has many different aspects to it.
Read on for a brief run-through of the most common methods of biological wastewater treatment methods.
Any company producing industrial waste typically has to incorporate some sort of wastewater treatment system to ensure compliance with their local environmental and waste discharge regulations.
The ideal wastewater treatment system will take into account the potential for environmental harm, the health of staff and the general public, the process or products which the facility is making or processing, and both upfront capital and ongoing operating costs.
Thinking about treating waste products before discharging any wastewater into the local drainage system will also keep operations on the right side of the law, and avoid the hefty fines often associated with breaching environmental legislation.
The simple version of the answer to this is that a typical biological wastewater treatment system uses bacteria and other microbes to clean contaminated water so that it passes predetermined standards.
The bacteria used in the processes uses the pollutants found in the wastewater as food.
As it consumes the pollutants it begins to create particles which start to stick together to create larger clumps.
This process allows the organic matter to eventually settle out of the wastewater solution, producing a sludge which can be easily disposed of as a solid waste.
There are three main types of biological wastewater treatment:
Biological Oxygen Demand, or BOD, is the name of the measurement used to quantify the amount of dissolved oxygen needed by the anaerobic bacteria in the system to break down organic matter.
A high level of BOD means that there is a high level of biodegradable material in the water.
This can be caused by several things including industrial discharge pollutants, domestic sewage, or run-off from fertilizers.
If pollutant levels are very high, BOD can also remove the oxygen required by other aquatic life forms.
This can cause the death of fish and other aquatic life, accelerate the growth of blooms of harmful algae and result in serious damage to the ecosystem in the area where the untreated or poorly treated wastewater is discharged.
This potential for environmental damage is what drives the requirements to treat waste before final discharge.
The type of wastewater produced by your operation, coupled with the discharge requirements in your particular area will determine which type of biological treatment is needed, and in which order the treatments should be sequenced.
Biological treatment systems therefore take an existing biological process, and optimise this to make it easier to remove contamination in industrial wastewater.
The best systems can replace, or be used alongside, other physical and chemical wastewater treatment processes.
The exact composition of a biological wastewater treatment system will depend on the chemical composition of the raw wastewater and might be made up several different steps.
The system will also include procedures which can keep biomass growth in check.
For example, engineers will often keep an eye on and adjust aeration to keep dissolved oxygen levels constant and at the correct rates to keep the system running efficiently.
As well as monitoring dissolved oxygen levels, operators have to balance other aspects of the system such as nutrients, temperature, flow and pH levels.
Trying to balance a wide range of factors in a biological treatment process can become very complex, very quickly.
There are several key technologies in used in the treatment of wastewater and these are explored below.
Aerobic wastewater treatment technologies
Common types of aerobic treatment technologies.
Activated sludge is a system that has been around since the start of the 20th century.
It’s the most common biological treatment used in large utility type water treatment plants but also has a place in other industrial settings.
Wastewater first flows into an aeration tank, where oxygen is pumped into the water to feed the freely-floating bacteria and other microorganisms.
These then break the organic material down to form biological solids which form clumps known as flocs.
Flocs can then be removed from the wastewater through the sedimentation process.
The disadvantage of activated sludge processes is that they require a lot of space, and can produce large amounts of sludge.
On the plus side, they are cheap to build and maintain when compared with other options.
Fixed-bed bioreactors or FBBRs are a technology that was developed in the 1970s and 80s.
FBBRs comprise a series of tanks with multiple chambers, packed with a porous material such as ceramic, foam or plastic.
The wastewater flows through the various chambers, with contaminants being eaten by microbes along the way.
The design of the chambers and porous material allows FBBRs to hold more microbes in the same space, making this a space-saving technology when compared with other options.
It’s also energy efficient, and ideal for treating wastewater at all BOD levels.
Sludge disposal costs are low and FBBRs have a long lifespan too.
Water can flow through the system without plugging or channeling.
There’s also the option to add other biological processes such as nitrification or desalination into the chambers, and the set-up of the system can be tailored for your specific requirements.
Moving-bed bioreactors or MBBRs are a Norwegian invention which came into use in the late 1980s.
This technology is now being used in many countries for treating both domestic wastewater and industrial wastewater.
A typical MBBR set-up comprises aeration tanks filled with small biofilm carriers of different shapes and sizes.
As the biofilm carriers are MBBRs are suspended and moving, this allows high BOD wastewater to be treated in a smaller area.
There’s usually a second stage in the process after a MBBR is used.
Excess sludge settles into a slurry which can be removed by vacuum, or solids can be pressed into solids for removal and disposal.
MBBRs are typically used in the first step of the treatment process, or used in situations where the quality of the effluent is of lesser importance.
MBBRs are typically used to treat wastewater from food and drink factories, meat processing or packing plants, oil refineries or petrochemical sites.
Membrane bioreactors or MBRs were developed in the 1990s.
Membrane modules are submerged into the aeration tank, and air is used to scour the submerged membrane to keep them clear.
This is an advanced treatment method which combines conventional activated sludge and membrane filtering to remove solids rather than depending on sedimentation.
MBRs produce a higher quality of effluent when compared with conventional activated sludge plants, and take up far less space.
The design of the system will depend on the type of wastewater produced and what type of end result is required.
A typical MBR will have both aerobic and anaerobic treatment tanks, a system for aeration, a tank with a membrane, and an ultrafiltration membrane.
Although effective, the downside of a MBR system is that it is expensive to build initially, and also involves higher operating and maintenance costs.
Biological trickling filters can be used to remove organic contamination from either wastewater or air.
The air or water is passed through some type of medium which will allow biofilm to collect on its surface.
This biofilm, composed of both anaerobic and aerobic bacteria, will break down organic contamination.
Gravel, foam, sand or ceramics might be used to create these systems.
These filters are more commonly found in water treatment plants but can be very effective in any situation where keeping smells to a minimum is essential.
The IFAS, an emerging technology, enhances treatment by adding a growth media in an activated sludge tank. This method boosts biomass and can modernize existing facilities without major construction.
The strength of IFAS is that it is the refinement of the advanced sludge method. It can therefore be easily integrated into existing installations.
In addition, adding a crib that can be either fixed or mobile provides many advantages:
IFAS, or “Integrated Fixed-Film Activated Sludge,” is a recent innovation for wastewater treatment. While inspired by traditional activated sludge methods, it is more effective. The main distinction between IFAS and MBBR lies in how the activated sludge is reused. This technology provides a modern and advantageous approach to water purification.
IFAS is a technology adapted to existing activated sludge installations in biological treatment. It has the advantage of using more compact tanks. These tanks are specially designed to accommodate media, whether dispersed or fixed. If you opt for dispersed media, consider adding additional screens for better efficiency.
This IFAS technology has many advantages over existing technologies. However, before installing an IFAS system in an existing station, it is important to make a few observations:
SBRs (sequential batch reactors) use a separate pretreatment section to mechanically retain the solids. Together with this, there is an aeration and biological settling tank.
Small SBR wastewater treatment systems clean the wastewater. This is a few sentences explaining how the SBR technology works.
In absence of oxygen, we can continue a process with maintaining systematic design;
The biogas, a mix of methane and carbon dioxide, is burned off, or can be used to generate electricity for using elsewhere in the plant.
Anaerobic digesters can break down organic waste without the need for oxygen.
This process is most commonly used in industrial treatment / sewage treatment, and frequently found digesters include covered lagoons, suspended or submerged media, stirred tank reactors and fixed film.
The MBBR process utilizes floating plastic carriers (media) within the aeration tank to increase the amount of microorganisms available to treat the wastewater. The microorganisms consume organic material. The media provides increased surface area for the biological microorganisms to attach to and grow in the aeration tanks. The increased surface area reduces the footprint of the tanks required to treat the wastewater. The media is continuously agitated by bubbles from the aeration system that adds oxygen at the bottom of the first compartment of the aeration tank. The microorganisms consume organic material. When compared to conventional secondary treatment it provides superior efficiency and value.
In fact the MBBR media have an active surface area > 500 m2/m3.
The MBBR is a complete mix, continuous flow through process which is based on the biofilms principle that combines the benefits of both the activated sludge process and conventional fixed film systems without their disadvantages.
The IFAS, an emerging technology, enhances treatment by adding a growth media in an activated sludge tank. This method boosts biomass and can modernize existing facilities without major construction.
The strength of IFAS is that it is the refinement of the advanced sludge method. It can therefore be easily integrated into existing installations.
In addition, adding a crib that can be either fixed or mobile provides many advantages:
IFAS, or “Integrated Fixed-Film Activated Sludge,” is a recent innovation for wastewater treatment. While inspired by traditional activated sludge methods, it is more effective. The main distinction between IFAS and MBBR lies in how the activated sludge is reused. This technology provides a modern and advantageous approach to water purification.
IFAS is a technology adapted to existing activated sludge installations in biological treatment. It has the advantage of using more compact tanks. These tanks are specially designed to accommodate media, whether dispersed or fixed. If you opt for dispersed media, consider adding additional screens for better efficiency.
This IFAS technology has many advantages over existing technologies. However, before installing an IFAS system in an existing station, it is important to make a few observations:
Membrane bioreactors or MBRs were developed in the 1990s.
Membrane bioreactor (MBR) technology is an efficient hybrid of traditional biological wastewater treatment and modern membrane processes that is used in both municipal and industrial wastewater treatment. As in legacy activated sludge processes, it uses microorganisms to degrade organic pollutants, but instead of using a bulky clarifier, it uses advanced membranes to reject suspended solids.
MBR technology is widely used across various sectors, including municipal wastewater treatment plants, industrial facilities, and decentralized treatment systems. Its versatility makes it suitable for applications ranging from small-scale to large-scale projects.
The MBR process begins with a pretreatment stage to minimize membrane fouling. Then, aerobic microorganisms break down organic matter in the MBR tank. An aeration system delivers air bubbles to the tank to keep the bacteria active as they break down organic pollutants.
A pumping system mixes water in the bioreactor tank and moves it through a module, where semipermeable membranes filter out suspended solids and microorganisms. MBR membranes are fibers or flat sheets made of polyethylene or polyvinylidene fluoride.
Chemical or backwash cleaning systems are used to minimize biofilm fouling of the membrane, supporting performance and long life. To save water, a backwash recovery system can be used to collect and recover the cleaning backwash water.
During the process, sensors and monitoring devices keep track of effluent parameters in real time as a control system simultaneously adjusts operational settings to optimize treatment.
MBRs generate sludge as a byproduct of the biological treatment process. A sludge management system separates and handles the excess sludge, which may be treated further or dewatered for efficient disposal.
Finally, a system collects the resulting permeate once it passes through the membrane.
Membrane bioreactor systems offer many advantages over legacy processes. They include:
The treatment of different effluents varies with the type of effluent. Wastewater enters the effluent or sewage treatment plant and goes through several processes before effluent goes into the environment. Industrial effluent treatment plant process include the following stages:
Electrocoagulation (EC) is a wastewater treatment technology capable of removing suspended solids, dissolved organic matter and nutrients, faecal indicator bacteria as well as heavy metals, oils and other organic contaminants. EC has been most widely used for the treatment of industrial wastewater, including textile, oil, paper, and dye wastewaters.
EC generates coagulants in situ by electrolytic oxidation of metal anodes. Iron or aluminium plates are commonly used for the anodes, releasing iron (Fe2+) and aluminium (Al3+) ions into the wastewater which hydrolyse to polymeric hydroxides. Polymeric hydroxides are excellent coagulants for the removal of various wastewater pollutants. Coagulation involves charge neutralization of negatively charged contaminants followed by the formation of flocs that either settle or float. Therefore, a subsequent solids removal stage (e.g., clarifier or Dissolved Air Flotation) is required.
EC efficiency can be improved by optimizing operational parameters including: electrode spacing, electrode orientation, periodic electrode polarity reversal, current density (A/m2) and contact time. Particularly, the removal efficiency of TSS and particulate BOD (including algae which are negatively charged) by EC is mainly dependent on the amount of Fe2+ or Al3+ ions generated from the anode. Therefore, greater removal can normally be achieved at higher current density. Phosphate ions (PO43-) are neutralized by the polymeric metal hydroxides which also directly bind to suspended P contaminants. These then aggregate and settle with the flocculated solids. All nitrogen compounds can be removed to some degree by EC. For example, organic nitrogen is removed with the flocs of TSS. EC can promote inactivation of microorganisms including faecal coliforms and viruses by rupturing their membranes and then coagulating them into settleable flocs. Furthermore, EC can remove heavy metals as metal hydroxides and other organic compounds including pesticides and halogenated hydrocarbons.
Many laboratory-scale EC trials have been conducted to determine optimum design and operation parameters for efficient wastewater treatment. However, currently there is no information on full-scale application of EC technology available in peer-reviewed scientific literature. This study tested a laboratory-scale EC unit for the treatment of wastewater pond effluent. Pond water samples (~20 L) were collected from an oxidation pond on three occasions and each sample was used on the same day for the laboratory experiments. The effect of different EC currents (between 0.4A and 3A) on the water quality of the wastewater pond effluent was investigated in terms of the removal of organic matter (TSS and BOD5), nutrients (nitrogen and phosphorus), and faecal coliforms. Physico-chemical parameters including temperature, pH, dissolved oxygen (D.O.), conductivity, turbidity and %UV transmittance (UVT) were also measured before and after the EC treatment.
This study showed that the laboratory-scale EC unit typically achieved >90% removal of TSS, BOD5 and TP, >95% removal of DRP, 50-80% removal of TKN, and 2-3 log removal of E. coli at a EC current of 0.8-1.6A. Full-scale EC unit power consumption would be ~0.4 kWh/m3 (Approx) wastewater. This research indicates that EC is an efficient and potentially cost-effective option for treating wastewater pond effluent since the EC can provide a combined removal of organic matter, phosphorus and disinfection (replacing chemical flocculation/coagulation and UV treatment) and produce a readily dewater able sludge.
Electrocoagulation treats water without the need for chemicals. This means there is no danger of residual chemicals making their way into the effluent, leaving behind toxins and odors. There is also no thickener required as there would be in chemical coagulation, which reduces the cost of the operation up front.
Research from 2009 found that electrocoagulation reduces the total number of suspended solids in the solution by as much as 95 to 99 percent.
The metals that are found suspended in untreated water may not be useless. In fact, they may be very useful indeed; it is just that they pose a hazard when they are suspended in water.
Some water treatment methods are unable to extract metals in a meaningful way, and instead destroy or remove them in other ways. Electrocoagulation is a little different.
As the process uses a form of electrolysis to separate and coagulate liquids, it can collect metals in a purer form. These metals can then be used in various applications.
It actually does not take much current, in most cases, to run the coagulation equipment. This low level of current can easily be produced using green energy sources.
Electrocoagulation requires electrodes to feed the current into the solution. Unfortunately, the process of coagulation is an intensive one, and places a lot of strain on the electrodes themselves, resulting in wear and tear.
This means, regular cleaning and maintenance for the electrodes are involved in the process. This can be labor-intensive work, not to mention expensive.
This also means a short life span for the electrodes, which need to be replaced often.
All of the following factors can affect the results of the process:
Chemical treatment of wastewater uses chemical reactants to break down pollutants. It is ideal for wastewaters with high chemical toxin contents, such as the wastewaters from chemical or pharmaceutical manufacturing, pulp and paper mills, laboratories and textile manufacturers. It is also optimal for removing the heavy metals found in mining wastewater. The chemical effluent treatment plants (ETPs) that treat these wastes use chemical treatment methods.
Wastewaters that contain toxic substances, especially toxic soluble metals, generally require chemical treatments. Any industry that uses raw materials from the earth — including industries like mining, steel manufacturing, and oil and gas extraction and refining — will produce wastewater with toxic soluble metals. These industries are more likely to use chemical treatment.
Below are a few of the common contaminants chemical waste management treatments can remove:
Chemical treatment may be a straightforward or complicated process depending on the contaminants in the water and the chemical reactions that take place. Most wastewater chemical treatment involves the following steps:
Depending on the contaminants, the wastewater may undergo intermediate steps, such as pH adjustments to enhance coagulation or temperature adjustments to optimize chemical reactions.
Microfiltration is a filtration process using a microporous media that retains the suspended solids of a fluid. The pore size of the membrane ranges from 0.1 to 1 micron or microns.
Microfiltration is different from reverse osmosis and nanofiltration in that it does not require pressure. It is often used as a pretreatment for reverse osmosis or as a stand-alone filtration process.
Microfiltration is a filtration process using a micrometer-sized (μm) filter. Filters can be at atmospheric pressure or with a vessel at a certain pressure (maximum 25 psi) (2), but they usually work at low pressures.
These filters are porous and allow water to pass through them, removing organic matter, suspended solids, small colloids, bacteria and turbidity.
Microfiltration is mainly used as a pretreatment step in the production of drinking water and industrial water. It has excellent properties to eliminate suspended solids, bacteria and cysts. It is an alternative to the classic sand filter. In addition, Crossflow MF is used for:
Microfiltration has many fields of application, such as:
MF is also used for wastewater reuse. In this case, it can be combined with a biostage in a membrane bioreactor.
CleanWater BD has own expertise about effluent treatment plant design, installation, about microfiltration, we have supporting foreign partners, but about installation, CleanWater BD are capable enough to complete the project;
Reverse Osmosis technology removes most contaminants from water by pushing the water through a semi-permeable membrane under pressure. This article provides an overview of Reverse Osmosis (RO) technology and its applications.
Reverse osmosis, often abbreviated as RO, removes a significant portion of dissolved solids and other contaminants from water by forcing it through a semi-permeable membrane.
To understand RO’s purpose and process, first you must understand the naturally occurring process of Osmosis.
Osmosis is a naturally occurring phenomenon, and one of the most important processes in nature, where a weaker saline solution will tend to migrate to a strong saline solution. For example, when plant roots absorb water from the soil, or our kidneys absorb water from our blood.
The diagram to the right shows how osmosis works.
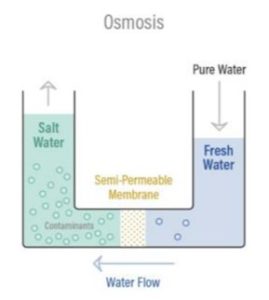
A less concentrated solution has a natural tendency to migrate to a solution with a higher concentration.
For example, if you had a container full of water with low salt concentration and another container full of water with high salt concentration, separated by a semi-permeable membrane, the water with the lower salt concentration would start to move towards the container with the higher salt concentration.
A semi-permeable membrane allows some atoms or molecules to pass but not others. A simple example is a screen door which allows air molecules to pass through but not pests or anything larger than the screen holes. Another example is Gore-Tex clothing which has an extremely thin plastic film with billions of small pores just big enough to let water vapor through, but small enough to prevent liquid water from passing.
RO is the process of Osmosis in reverse. Osmosis occurs naturally without an external energy source, but reversing the osmosis process requires applying energy to the more saline solution to reverse the natural flow.
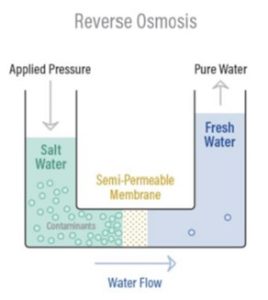
A reverse osmosis membrane is a semi-permeable membrane that allows the passage of water molecules but not most of the dissolved salts, organics, bacteria, and pyrogens. However, the water must be “pushed” through the RO membrane by applying pressure greater than the naturally occurring osmotic pressure.
The diagram on the right outlines the RO process.
When pressure is applied to the concentrated solution, the water molecules are forced through the semi- permeable membrane while the contaminants are not allowed through.
RO works using a high-pressure pump to apply pressure on the salt side of the RO system and to force the water across the semi- permeable RO membrane, leaving almost all (95% to 99%) of dissolved salts behind in the reject stream.
The amount of pressure required depends on the salt concentration of the feed water. The more concentrated the feed water, the more pressure is required to overcome the osmotic pressure.
In very simple terms, feed water is pumped into an RO system and two types of water come out: good water (permeate) and bad water (concentrate).
The “good” water has most contaminants removed and is called permeate. Another term for permeate is product water. Permeate is the water that was pushed through the RO membrane to remove nearly all contaminants. RO system sizes are based on permeate flow. For example, a 100 gallon per minute (gpm) RO system will produce 100 gpm of permeate water.
The “bad” water, called the concentrate, reject, or brine, is the leftover liquid will all the contaminants unable to pass through the RO membrane. All three terms are used interchangeably and mean the same thing. The simple schematic below shows how water flows through an RO system.
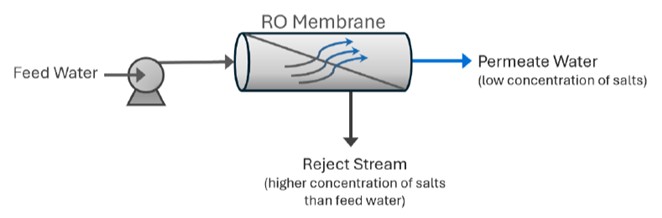
As the feed water enters the RO membrane under pressure (enough to overcome osmotic pressure) the water molecules pass through the semi-permeable membrane and the salts and other contaminants remain on the other side and are discharged from the system through the concentrate stream.
The concentrate either goes to a drain or, in some circumstances, is fed back into the feed water supply and recycled through the RO system to save water. The water that makes it through the RO membrane usually has approximately 95% to 99% of dissolved salts removed.
It is important to understand that RO systems employ cross filtration rather than standard dead-end filtration in which contaminants are collected within the filter media. With cross filtration, the solution passes through, or crosses, the filter with two outlets routing the filtered water one way while the contaminated water goes a different route. Cross flow filtration allows water to sweep away contaminant build up and allow enough turbulence to keep membrane surfaces clean.
RO can remove 95-99% of dissolved salts (ions), particles, colloids, organics, bacteria, and pyrogens from feed water. An RO membrane rejects contaminants based on their size and charge. Any contaminant with a molecular weight greater than 200 will likely be rejected by a properly running RO system.
The greater the ionic charge of the contaminant, the more likely it will be unable to pass through the RO membrane. For example, a sodium ion has only one charge (monovalent) and is not rejected by the RO membrane as well as calcium, which has two charges.
RO systems cannot remove dissolved gases, such as carbon dioxide (CO2), very well because they are not highly ionized (charged) while in solution and have a very low molecular weight. Because RO systems do not remove gases, permeate water can have a slightly lower than normal pH level, depending on dissolved CO2 in the feed water since CO2 is converted to carbonic acid.
RO is very effective in treating brackish, surface and ground water for both large and small flow applications. Industries that use RO water include pharmaceutical, boiler feed water, food and beverage, metal finishing, and semiconductor manufacturing to name a few.
There are a handful of calculations that are used to judge the performance of an RO system and for design considerations. An RO system has instrumentation that displays quality, flow, pressure and sometimes other data like temperature or hours of operation. To accurately measure the performance of an RO system you need the following operation parameters at a minimum:
This equation tells you how effectively the RO membranes are removing contaminants. It does not tell you how each individual membrane is performing. However, it will tell you how the system overall is performing on average.
A well-designed RO system with properly functioning RO membranes will reject 95% to 99% of most feed water contaminants (of a certain size and charge).
The following equation can be used to determine how effective the RO membranes are at removing contaminants:
Salt Rejection % = ((Feed water conductivity – Permeate water conductivity) / Feed water conductivity) x 100
This is simply the inverse of salt rejection described in the previous equation. This is the amount of salts, expressed as a percentage, passing through the RO system.
The lower the salt passage, the better the system is performing. A high salt passage can mean the membranes require cleaning or replacement.
Salt Passage % = (1 – Salt Rejection %) x 100
Recovery is the amount of feed water emerging from the system as good, permeate water.
Another way to think about recovery is as the amount of water collected as permeate or product water instead of being sent to drain as concentrate. Higher recovery percents mean you are sending less water to drain as concentrate and saving more permeate water. However, if recovery percents are too high for the RO design, it can lead to larger problems from membrane scaling and fouling.
Design software establishes RO system recovery rates by considering numerous factors such as feed water chemistry and pre-treatment before the RO system. Therefore, proper RO system recovery depends on the design. Calculating the recovery facilitates rapid determination that the system is operating outside of the intended design.
The calculation below expresses the recovery rate as a percentage.
% Recovery= (Permeate Flow Rate gpm / (Permeate Flow Rate gpm + Concentrate Flow Rate gpm)) x 100
For example, if the recovery rate is 80% then for every 100 gallons of feed water entering the RO system, you are recovering 80 gallons as usable permeate water while 20 gallons go to the drain as concentrate. Industrial RO systems typically run between 50% to 85% recovery depending on feed water characteristics and other design considerations.
The concentration factor is related to RO system recovery and is important for RO system design. The more water you recover as permeate (higher recovery %), the more concentrated salts and contaminants you collect in the concentrate stream. When the concentration factor is too high for the system design and feed water composition, the system may experience increased scaling on RO membrane surfaces.
Concentration Factor = (1 / (1 – Recovery %)
The concept is no different than that of a boiler or cooling tower with purified water exiting the system as steam leaving a concentrated solution behind. As the concentration increases, solubility limits may be exceeded and precipitate on equipment surfaces as scale.
For example, if your feed flow is 100 gpm and the permeate flow is 80 gpm, then the recovery is (80/100) x 100 = 80%.
To find the concentration factor, the formula would be 1 ÷ (1 – 80%) = 5.
A concentration factor of 5 means the water going to the concentrate stream will be 5 times more concentrated than the feed water. If the feed water in this example was 500 ppm, then the concentrate stream would be 500 x 5 = 2,500 ppm.
Flux expresses the amount of water passing (permeating) through a reverse osmosis membrane during a given time, measured as gallons per square foot per day (GFD) or liters per square meter per hour (l/m²/hr).
A higher flux means more water is permeating through the RO membrane.
Designers establish RO systems to operate within a specific flux range to ensure that the water flowing through the RO membrane is neither too fast nor too slow.
Flux Gfd = (Permeate flow gpm * 1,440) / (# of RO elements in system * square footage of each RO element)
For example, you have the following:
Gfd= (75 gpm X 1,440) / (18 x 400)
= 16 Gfd
This means 16 gallons of water passed through each square foot of each RO membrane per day.
This number could be good or bad depending on the feed water chemistry and system design. Below is a general rule of thumb for flux ranges for different source waters. This can be better determined with the help of RO design software.
RO Permeate Water 20-30
Brackish Well Water 14-18
Brackish Surface Water 10-14
Sea Water 8-12
Sewage Effluent 5-10
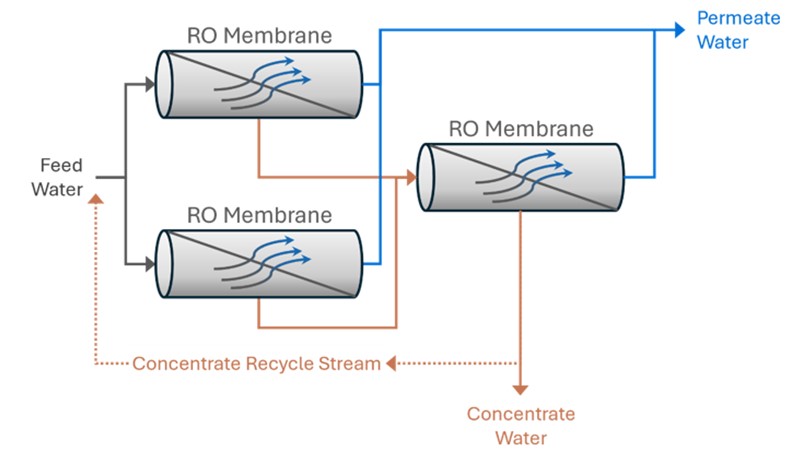
The term ‘stage’ and ‘pass’ are often mistaken for the same thing in an RO system, and the terminology can be confusing for an RO operator. It is important to understand the difference between a one- and two-stage RO and a one- and two-pass RO.
Difference between a One and Two Stage RO System
In a one-stage RO system, the feed water enters as one stream and exits the RO as either concentrate or permeate water.
In a two-stage system, the concentrate (or reject) from the first stage then becomes feed water for the second stage. The permeate water collected from the first stage is combined with permeate water from the second stage. Additional stages increase the RO system’s recovery.

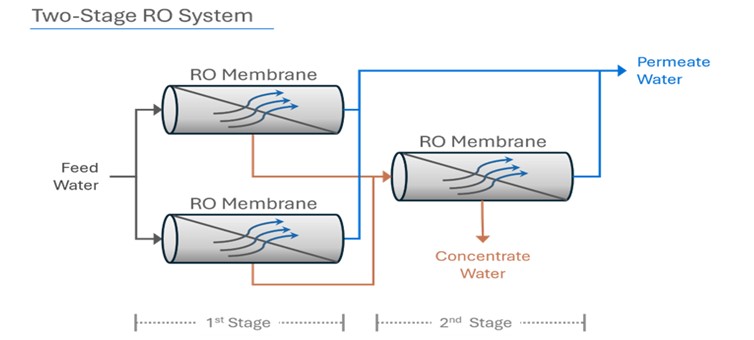
In a reverse osmosis system, an array describes the physical arrangement of the pressure vessels in a two-stage system. Pressure vessels contain RO membranes (usually from 1 to 6 RO membranes are in a pressure vessel), and each stage can have a certain amount of pressure vessels with RO membranes.
The reject of each stage then becomes the feed stream for the next successive stage. The two-stage RO system above is a 2:1 array, which means the concentrate (or reject) of the first two RO vessels is fed to the next single vessel.
If an RO system cannot be properly staged and the feed water chemistry permits, you can use a concentrate recycle setup where a portion of the concentrate stream is fed back into the feed water of the first stage to enhance system recovery.
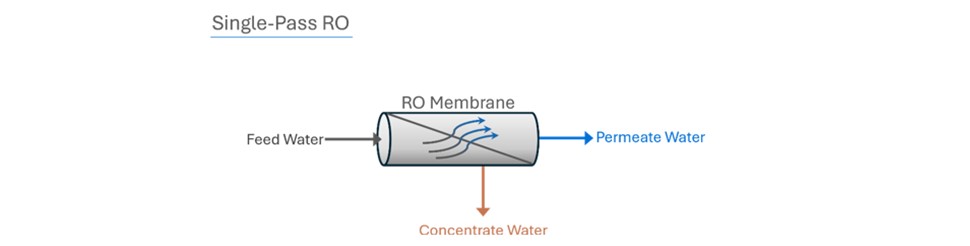
Think of a ‘pass’ as a standalone RO system. The difference between a single-pass RO system and a double- pass RO system is how many RO systems the water passes through.
In a double-pass RO, the permeate from the first RO (the first pass) becomes the feed water to the second pass (or second RO). A double-pass RO system produces a much higher quality permeate because it has essentially gone through two RO systems.
In addition to producing a much higher quality permeate, a double-pass system also provides the opportunity to remove carbon dioxide gas from the permeate by injecting caustic between the first and second pass. C02 is undesirable when using mixed bed ion exchange resin beds after the RO system.
Adding caustic after the first pass raises the pH of the first pass permeate water and converts CO2 to bicarbonate (HCO3) and carbonate (CO3-2), which RO membranes in the second pass reject more effectively.
This process is not feasible with a single pass RO system because injecting caustic and forming carbonate (CO3-2) in the presence of cations like calcium leads to scaling of RO membranes.

Proper pretreatment using both mechanical and chemical treatments is critical for an RO system to
prevent fouling, scaling and costly premature RO membrane failure and frequent cleaning requirements. Below is a summary of common problems an RO system experiences due to lack of proper pretreatment.
Fouling occurs when contaminants accumulate on the membrane surface effectively plugging the membrane. Many contaminants in municipal feed water are naked to the human eye and harmless for human consumption. However, they are large enough to quickly foul (or plug) an RO system.
Fouling typically occurs in the front end of an RO system and results in a pressure drop across the RO system and a lower permeate flow. This translates into higher operating costs and eventually the need to clean or replace the RO membranes.
Fouling will take place eventually due to an RO membrane’s extremely fine pore size no matter how effective the pretreatment protocols or cleaning schedule. However, proper pretreatment will minimize the need to address fouling related problems.
The following can cause fouling:
Bacteria are one of the most common fouling problems. This is because RO membranes cannot tolerate disinfectants such as chlorine and microorganisms are often able to thrive and multiply on the membrane surface. Microorganisms may produce biofilms that cover the membrane surface and result in heavy fouling.
Filter media upstream of the RO unit breakthrough may involve GAC carbon beds and softener beds developing an under-drain leak. Without adequate post filtration, the media can foul the RO system.
Analytical tests determine if the feed water to your RO has a high potential for fouling. mechanical filtration helps prevent RO system fouling. The most popular methods to prevent fouling are the use of multi-media filters (MMF) or microfiltration (MF). In some cases, cartridge filtration will suffice.
As certain dissolved (inorganic) compounds become more concentrated (remember discussion on concentration factor) scaling can occur. If these compounds exceed their solubility limits, they can precipitate on the membrane surface as scale. Scaling causes higher pressure drops across the system, higher salt passage (less salt rejection), and low permeate flow.
Common scale that tends to form on RO membranes is calcium carbonate (CaCO3).
Modern thin film composite membranes are not tolerant to chlorine or chloramines. Oxidizers, such as chlorine, will ‘burn’ holes in the membrane pores and can cause irreparable damage. The result of chemical attack on an RO membrane is higher permeate flow and higher salt passage (less salt rejection).
Increased microorganism growth on RO membranes tends to easily foul membranes since there is no biocide present to prevent growth.
Part of the pretreatment scheme should involve pre and post RO system plumbing and controls. If ‘hard starts’ occur, the system may experience mechanical damage to the membranes. Likewise, too much backpressure on the RO system can cause mechanical damage to the RO membranes.
These can be addressed by using variable frequency drive motors to start high pressure pumps for RO systems along with installing check valve(s) and/or pressure relief valves to prevent excessive back pressure on the RO unit that can cause permanent membrane damage.
The pretreatment solutions for RO systems listed below can help minimize fouling, scaling and chemical attack.
Multi-Media Filters help prevent RO systems from fouling. An MMF typically contains three layers of media consisting of anthracite coal, sand, and garnet, with a supporting gravel layer at the bottom. These are the medias of choice because of the differences in size and density. The larger (but lighter) anthracite coal will be on top, and the heavier (but smaller) garnet will remain on the bottom.
The filter media arrangement removes the largest dirt particles near the top of the media bed and retains smaller dirt particles deeper within the media. The entire bed acts as a filter allowing much longer filter run times between backwashes and more efficient particulate removal.
A well-operated MMF can remove particulates down to 15 – 20 microns. An MMF with an incorporated coagulant removes particulates down to 5 – 10 microns by inducing tiny particles to join and form larger particles that can be filtered. To put this in perspective, the width of a human hair is around 50 microns.
When the feed water Silt Density Index (SDI) value exceeds 3 or when turbidity exceeds 0.2 NTU, experts recommend using a multi-media filter. While there’s no exact rule, following these guidelines helps prevent premature fouling of RO membranes.
It is important to have a 5-micron cartridge filter placed directly after the MMF unit to prevent the MMF media from damaging downstream pumps and fouling the RO sustem if the MMF under drains fail.
Microfiltration (MF) is effective in removing colloidal and bacteria matter with a 0.1-10µm pore size and is helpful in reducing RO unit fouling potential. Membrane configuration can vary between manufacturers, but the “hollow fiber” type is the most common.
Typically, pumps draw water from the outside of the fibers, while clean water collects inside the fibers. Microfiltration membranes used in potable water applications usually operate in “dead-end” flow. Specifically, all the water fed to the membrane is filtered through the membrane. Periodically backwash the installed filter cake to remove it from the membrane surface.
Recovery rates are normally greater than 90 percent on feed water sources with high quality and low turbidity feeds.
Antiscalants and scale inhibitors, as their name suggests, are chemicals added to feed water before an RO unit to help reduce the scaling potential. Antiscalants and scale inhibitors increase the solubility limits of troublesome inorganic compounds.
By increasing the solubility limits, you can concentrate the salts further than otherwise would be possible, achieving a higher recovery rate and operating at a higher concentration factor.
Antiscalants and scale inhibitors work by interfering with scale formation and crystal growth. The choice of antiscalant or scale inhibitor and correct dosage depends on feed water chemistry and RO system design.
A water softener can be used to help prevent RO system scaling. Water softeners exchange scale forming ions with non- scale forming ions. As with MMF units, it is important to have a 5-micron cartridge filter placed directly after the water softener if the under drains fail.
Sodium bisulfite (SBS or SMBS), a reducer, added to the water stream before an RO at the proper dose can remove residual chlorine and chloramines.
GAC removes both organic constituents and residual disinfectants (such as chlorine and chloramines) from water. Manufacturers make GAC media from coal, nutshells, or wood. Activated carbon removes residual chlorine and chloramines by a chemical reaction. It involves a transfer of electrons from the surface of the GAC to the residual chlorine or chloramines. The chlorine or chloramines ends up as a chloride ion that is no longer an oxidizer.
The disadvantage of using a GAC before the RO unit is that the GAC will remove chlorine quickly at the very top of the GAC bed. This will leave the remainder of the GAC bed without any biocide to kill microorganisms. A GAC bed will absorb organics throughout the bed, which is potential food for bacteria. Eventually, the GAC bed can become a breeding ground which can pass easily to the RO membranes.
Also, a GAC bed can produce very small carbon fines under some circumstances that have the potential to foul an RO. Place a cartridge filter after GAC and before RO to protect membranes from carbon fines.
RO membranes are the heart of the RO system. It is important to collect certain data points to determine its health. These data points include system pressures, flows, quality, and temperature.
Water temperature is directly proportional to pressure. As the water temperature decreases it becomes more viscous. Thus, the RO permeate flow will drop as more pressure is required to push the water through the membrane. Likewise, when the water temperature increases, the RO permeate flow will increase. Normalize RO system performance data to prevent mistaking flow variations for abnormalities when no actual problem exists.
Calculate, graph, and compare normalized flows, pressures, and salt rejection to baseline data. Obtain baseline data when commissioning the RO or after cleaning or replacing the membranes. This helps you troubleshoot problems and decide when to clean or inspect the membranes for damage.
Data normalization helps show the RO membranes’ true performance. As a general rule, investigate the cause and clean membranes when there is a normalized change of +/- 15% from baseline data. Otherwise, RO membrane cleanings may not be effective at bringing the membranes back to near new performance.
RO membranes will inevitably require periodic cleaning – usually between 1 to 4 times a year depending on feed water quality. Generally, if the normalized pressure drops or the normalized salt passage has increased by 15%, it is time to clean the RO membranes. Or if the normalized permeate flow has decreased by 15% then it is also time to clean the RO membranes.
The RO membranes can be cleaned in place (if equipped) or removed from the RO system and cleaned off site by a specialized service company. Offsite membrane cleaning delivers more effective cleaning than onsite cleaning skids.
RO membrane cleaning involves low and high pH cleaners to remove contaminants from the membrane. We address scaling with low pH cleaners and treat organics, colloidal matter, and biofouling with high pH cleaners.
Cleaning RO membranes is not only about using the appropriate chemicals. Many other factors, such as flows, water temperature, water quality, and properly designed and sized cleaning skids, require the involvement of an experienced service provider to clean RO membranes properly and prevent any damage that would necessitate replacement.
Reverse Osmosis is an effective and proven technology to reduce water contaminants.
Further post treatment after the RO system, such as mixed bed deionization, can increase RO permeate quality and make it suitable for the most demanding applications. Proper pretreatment and RO system monitoring are crucial to preventing costly repairs and unscheduled maintenance.
With the correct system design, maintenance program, and experienced service support, your RO system should provide high purity water for many years.
CleanWater BD has own expertise about effluent treatment plant design, installation, about RO system design, we have supporting foreign partners, but about installation, CleanWater BD are capable enough to complete the project;
Zero liquid discharge (ZLD) is an engineering approach to water treatment where all water is recovered and contaminants are reduced to solid waste.
While many water treatment processes attempt to maximize the recovery of freshwater and minimize waste, ZLD is the most demanding target because the cost and challenges of recovery increase as the wastewater gets more concentrated. Salinity, scaling compounds, and organics all increase in concentration, which add costs associated with managing these increases. ZLD is achieved by stringing together water treatment technology that can treat wastewater as the contaminants are concentrated.
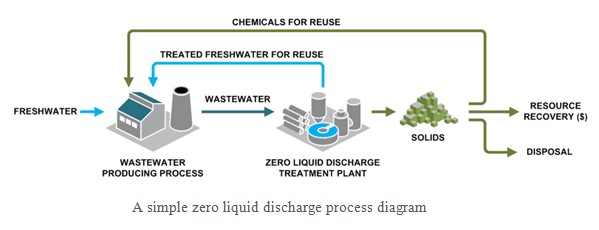
Targeting ZLD for an industrial process or facility provides a number of benefits:
The exact components of a ZLD treatment system will largely depend on (1.) the volume of dissolved material present in the waste, (2.) the system’s required flow rate, and (3.) what specific contaminants are present. But in general, a basic ZLD treatment system typically includes some type of:
Specific treatment processes vary, but a typical ZLD treatment facility process will usually include the following steps:
Pretreatment and conditioning
Pretreatment is used to remove simple things from the wastewater stream that can be filtered or precipitated out, conditioning the water and reducing the suspended solids, organic pollution loads and materials, that would otherwise scale and/or foul following treatment steps.
Typically this treatment block consists of some type of clarifier and/or a reactor to precipitate out metals, hardness, and silica. Sometimes this step requires the addition of caustic soda or lime to help with coagulation, a process where various chemicals are added to a reaction tank to remove the bulk suspended solids and other various contaminants. This process starts off with an assortment of mixing reactors, typically one or two reactors that add specific chemicals to take out all the finer particles in the water by combining them into heavier particles that settle out. The most widely used coagulates are aluminum-based such as alum and polyaluminum chloride.
Sometimes a slight pH adjustment will help coagulate the particles, as well.
When coagulation is complete, the water enters a flocculation chamber where the coagulated particles are slowly stirred together with long-chain polymers (charged molecules that grab all the colloidal and coagulated particles and pull them together), creating visible, settleable particles that resemble snowflakes.
The gravity settler (or sedimentation part of the ZLD treatment process) is typically a large circular device where flocculated material and water flow into the chamber and circulate from the center out. In a very slow settling process, the water rises to the top and overflows at the perimeter of the clarifier, allowing the solids to settle down to the bottom of the clarifier into a sludge blanket. The solids are then raked to the center of the clarifier into a cylindrical tube where a slow mixing takes place and the sludge is pumped out of the bottom into a sludge-handling or dewatering operation. The settlers can also be designed using a plate pack for smaller footprint.
Depending on the material in the feed, additional reactors, secondary treatment or chemistry may be required for the reduction of organic pollution loads, metals or silica. Careful consideration must be given to the pretreatment step for a successful ZLD system.
Ultrafiltration (UF) can also be used after the clarifiers instead of the gravity sand filter, or it can replace entire clarification process altogether. Membranes have become the newest technology for treatment, pumping water directly from the wastewater source through the UF (post-chlorination) and eliminating the entire clarifier/filtration train.
Out of this process comes a liquid that is then filter-pressed into a solid, resulting in a solution much lower in suspended solids and without the ability to scale up concentration treatment.
Phase-one concentration
Concentrating in the earlier stages of ZLD is usually done with membranes like reverse osmosis (RO), brine concentrators, or electrodialysis.
The RO train will capture the majority of dissolved solids that flow through the process, but as mentioned in a prior article about common problems with ZLD, it’s important to flow only pretreated water through the RO system, as allowing untreated water to go through the semipermeable membranes will foul them quickly. Brine concentrators, on the other hand, are also used to remove dissolved solid waste but they are usually able to handle brine with a much higher salt content than RO. They are pretty efficient for turning out a reduced-volume waste.
Electrodialysis can also be used at this part of the ZLD treatment system. It’s a membrane process that uses positively or negatively charged ions to allow charged particles to flow through a semipermeable membrane and can be used in stages to concentrate the brine. It is often used in conjunction with RO to yield extremely high recovery rates.
Combined, these technologies take this stream and concentrate it down to a high salinity while pulling out up to 60–80% of the water.
Evaporation/crystallization
After the concentration step is complete, the next step is generating a solid, which is done through thermal processes or evaporation, where you evaporate all the water off, collect it, and reuse it. Adding acid at this point will help to neutralize the solution so, when heating it, you can avoid scaling and harming the heat exchangers. Deaeration is often used at this phase to release dissolved oxygen, carbon dioxide, and other noncondensible gases.
The leftover waste then goes from an evaporator to a crystallizer, which continues to boil off all the water until all the impurities in the water crystallize and are filtered out as a solid.
Recycled water distribution/solid waste treatment
If the treated water is being reused in an industrial process, it’s typically pumped into a holding tank where it can be used based on the demands of the facility. The ZLD treatment system should have purified the water enough to be reused safely in your process.
The solid waste, at this point, will enter a dewatering process that takes all the water out of the sludge with filter or belt presses, yielding a solid cake. The sludge is put onto the press and runs between two belts that squeeze the water out, and the sludge is then put into a big hopper that goes to either a landfill or a place that reuses it. The water from this process is also typically reused.
CleanWater BD has own expertise about effluent treatment plant design, installation, about recycle and zero discharge plant design, are taking support from foreign partners, but about installation of recycle or ZLD plant, CleanWater BD are capable enough to complete the project;
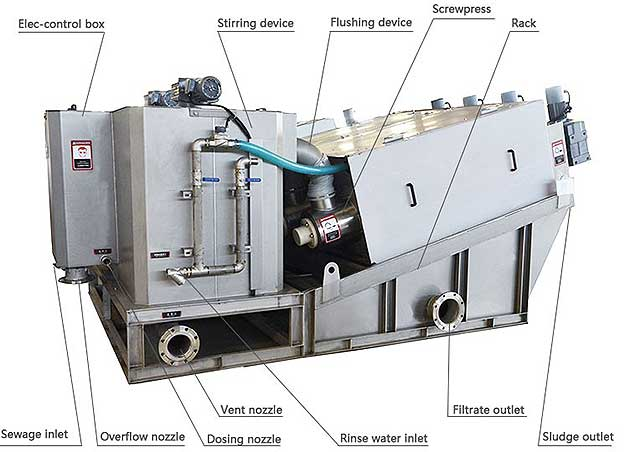
Sludge management consists of different distinct functions such as Solid Liquid separation, Sludge Thickening, Sludge De- watering, and Sludge disposal. The following Equipment some part design and some parts directly supplied by CleanWater play a significant role in the Sludge Management Process.
Sludge Dewatering System (Screw Press)
Filter Press


Multi effect evaporator is the most efficient and successful conventional evaporation method to achieve a complete zero-liquid discharge for any type of industrial effluent.
Prior to start evaporation process first cooling water circulation is started and generation of appropriate vacuum is being maintain by the vacuum pump, the purpose of the vacuum is to lower down the boiling point of the solution
Through feed pump the feed from the feed tank enters into the shell and tube heat exchanger called calandria by passing through the preheater.
Preheaters are used to exchange the sensible heat of condensate water generated during evaporation process to increase the initial temperature of the incoming feed, simultaneously decreasing the temperature of the condensate water which leads to decrease in the total energy requirement for the entire system
From the preheater effluent goes to the recirculation line of calandria-1 and enter into tubes of the bottom part of the calandria by the force provided by recirculation pump.
Feed passes through shell and tube heat exchanger in the tubes.
To start the evaporation process external steam is being provided to the shell side of the calandria for heating of the tubes.
Due to conductive heat transfer, steam passes its latent heat to effluent and evaporation of water from the effluent takes place. A water-vapor mixture is being generated which goes to Vapor-Liquid Separator called VLS.
In VLS, vapor is separated from water vapor mixture by means of gravity.
While the concentrated liquid is recirculated in the calandria by a recirculation pump.
Now the pure water vapor from VLS is either condensed in condenser or will be used as a heating medium in subsequent effect
This is the simple process of Single effect which uses 1 kg of steam to evaporate the 1 kg of water.
To increase steam economy, we can add 2nd effect, 3rd effect and up to 6 effect and so on.
The vapor of the 1st effect is used as a Heating Source for the 2nd effect and so on up to designed effect. thus, similarly vapor form the intial effect used as a heating medium for the further effect.
Thus, there is no steam is required individual effect except for the 1st effect.
To maintain the proper flow of vapor As we move further in the effects temperature and pressure are in decreasing manner.
Steam Economy statistics:
MEE principle can be continued over further effects to save even more energy.
Live Steam | Amount of Water To Be Evaporated | Energy Consumption | |
Single Effect Plant | 1 kg | 1 kg | 100 % |
Two Effect Plant | 1 kg | 2 kg | 50 % |
Three Effect Plant | 1 kg | 3 kg | 33 % |
Four Effect Plant | 1 kg | 4 kg | 25 % |
Five Effect Plant | 1 kg | 5 kg | 20 % |
This way, by adding no. of effects in the system we can increase the steam economy which leads to overall reduction in steam consumption and boiler capacity and thus in overall reduction in the operating cost of Zero Liquid Discharge.
From the bottom of the last effect, reject of the MEE at a certain concentration, goes to ATFD for final solid liquid separation, and thus achieving true mean of Zero Liquid Discharge.
Main Applications:
Features:
Products Features for MVR Falling Film Evaporator
CleanWater BD are taking support from foreign partners;
L
Mercerization is a finishing process in the textile industry where the textile fibers are treated under tensile stress with caustic soda. Large quantities of diluted caustic soda (weak lye) are a waste product of this process. Caustic Recovery Plants (CRP) can turn a very large proportion of this weak lye into reusable concentrated caustic soda (strong lye).
For the diverse requirements of our valued clients, we manufacture and supply the finest quality array of Caustic Recovery Plants. Developed in accordance with the quality standards, these plants are manufactured using premium quality raw material and advanced technology.
Caustic Soda Recovery Plants for Textile Industry separate the wash liquor from mercerizer into strong lye and vapor condensate. The condensate can be used for pre-washing and the caustic soda can be reused in the mercerizing process once again.
We are supplying 5 KLD, 10 KLD, 25 KLD, 50 KLD, 75 KLD, 100 KLD, 150 KLD, 200 KLD, 250 KLD & > 250 KLD CRP System in Textile Industries with the help of our foreign partner.
Market Segment || Clean Water BD Contact Us and see our social media like Facebook and YouTube for more information about this ETP plant.